IFCC Standardization of HbA1c
View/Print Entire Document (pdf)
Overview | The IFCC Reference System | Traceability and the EU Directive | The IFCC and NGSP
The IFCC and NGSP
IFCC or NGSP Numbers: The Debate
Figure 1 |
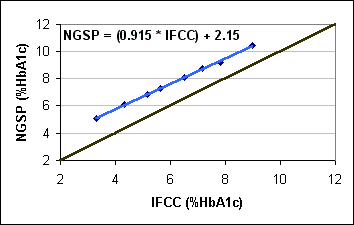 |
IFCC results are accuracy-based; NGSP results can be directly related to clinical outcomes and diabetes care goals. Although the IFCC/NGSP correlation is excellent, the absolute numbers are different and there has been much debate over which numbers should be reported worldwide. The relationship between the IFCC and NGSP (both in %HbA1c) is shown in Figure 1. IFCC results are consistently 1.5-2% HbA1c lower throughout the range of values.
The relationship between the NGSP network and IFCC networks was evaluated and a master equation was developed to document this relationship (NGSP = [0.9148 * IFCC] + 2.152). In 2007, the IFCC recommended that IFCC HbA1c be expressed as mmol HbA1c/mol Hb. With these new units, the master equation changes to NGSP = [0.09148 * IFCC] + 2.152). This change in units avoids any confusion between NGSP and IFCC results.
The master equation links IFCC results to clinically meaningful HbA1c results from the Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS). It also provides the NGSP with traceability to a higher order reference method.
In 2004, the ADA/EASD/IDF Working Group of the A1c Assay was established in 2004 to harmonize HbA1c reporting. The advantages and disadvantages of reporting HbA1c results in the new IFCC range vs. maintaining current NGSP/DCCT/UKPDS values, as well as reporting HbA1c as mean blood glucose, were discussed. It was agreed that further studies (over the next 3 years) would be done to investigate the feasibility of reporting HbA1c as mean blood glucose. The ADA, EASD and IDF sponsored a study (2006-2008) which was designed to better define the mathematical relationship between HbA1c and average glucose (AG). The study included 507 subjects with Type 1 and Type 2 diabetes and without diabetes from 10 international centers. Estimated average glucose (eAG) was calculated by combining weighted results from at least 2 days of continuous glucose monitoring performed four times, with seven-point daily self-monitoring of capillary glucose performed at least 3 days per week. The relationship between eAG and HbA1c based on linear regression analysis was: eAG(mg/dl)= (28.7*HbA1c)-46.7, r2=0.84. More information on the relationship between glucose and HbA1c can be found here.
Table 1 below depicts the relationships between NGSP and IFCC HbA1c as well as with eAG (mmol/L and mg/dL).
Table 1
NGSP HbA1c |
IFCC HbA1c (mmol/mol) |
eAG |
eAG |
5.0 |
31 |
97 |
5.4 |
6.0 |
42 |
126 |
7.0 |
7.0 |
53 |
154 |
8.6 |
8.0 |
64 |
183 |
10.2 |
9.0 |
75 |
212 |
11.8 |
10.0 |
86 |
240 |
13.4 |
11.0 |
97 |
269 |
14.9 |
12.0 |
108 |
298 |
16.5 |
Conversion factors for IFCC compared to each of the designated comparison methods (DCMs) including the NGSP are shown below in Table 2:
Table 2
DCM |
From IFCC to DCM |
From DCM to IFCC |
NGSP (USA) |
NGSP = (0.09148*IFCC) + 2.152 |
IFCC = (10.93*NGSP) - 23.50 |
JDS/JSCC (Japan) |
JDS = (0.09274*IFCC) +1 .724 |
IFCC = (10.78*JDS) - 18.59 |
Mono-S (Sweden) |
Mono-S = (0.09890*IFCC) + 0.884 |
IFCC = (10.11*Mono-S) - 8.94 |
Officially, there is worldwide consensus that HbA1c should be reported in both NGSP (%) and IFCC (mmol/mol) units along with eAG (in either mmol/L or mg/dL). However, the decision on what to report is actually being made country by country. The US will continue to report NGSP %HbA1c; the ADA and AACC have also recommended that estimated average glucose (eAG) be reported along with the HbA1c result. Some other countries have also decided and are reporting IFCC mmol/mol or both IFCC and NGSP. Although the world will again be reporting different numbers, results will be traceable to IFCC numbers as well as to clinical data through linear equations that are carefully monitored. The ADA, IDF, EASD, and ISPAD as well as other member associations in different countries currently provide patient care guidelines that relate directly to NGSP (DCCT/UKPDS) numbers. These will need to be updated to include both NGSP and IFCC numbers.
IFCC Network as an NGSP anchor
The IFCC network is now a secondary anchor for the NGSP. The stability of the relationship over time between the IFCC and NGSP will continue to be monitored. The NGSP certification process will not change and glycated hemoglobin (HbA1c) test results in the US will continue to be reported as %HbA1c (NGSP numbers) as recommended by the ADA and AACC
References:
Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I, Hoshino T, John WG, Kobold U, Little R, Mosca A, Mauri P, Paroni R, Susanto F, Takei I, Thienpont L, Umemoto M, Wiedmeyer HM. IFCC Reference System for Measurement of Hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan and Sweden: a method comparison study. Clin.Chem. 2004; 50: 166-174
Geistanger A, Arends S, Berding C, Hoshino T, Jeppsson J-O, Little R, Siebelder C and Weykamp C, on behalf of the IFCC Working Group on Standardization of HbA1c: Statistical Methods for Monitoring the Relationship between the IFCC Reference Measurement Procedure for Hemoglobin A1c and the Designated Comparison Methods in the United States, Japan and Sweden. Clin Chem 2008; 54(8): 1379-8
Report of the ADA/EASD/IDF Working Group of the HbA1c Assay, London, UK, January 2004. Diabetologia 2004;47:R53-4
Sacks DB for the ADA/EASD/IDF Working Group of the HbA1c Assay. Global harmonization of Hemglobin A1c. Clin Chem 2005;51:681-3
Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ for the A1c-Derived Average Glucose (ADAG) Study Group: Translating the hemoglobin A1c assay into estimated average glucose values. Diabetes Care 2008, 31:1473-8.