NGSP Protocol
 |
View/Print Entire Protocol (pdf)
Overview | Steering Committee | Laboratory Network | Certification | Proficiency Testing
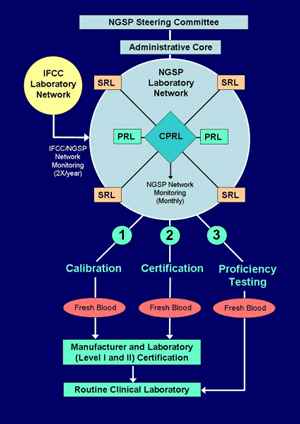
F: Proficiency Testing to Document Success of the HbA1c Standardization Program
Laboratories are asked to participate in the GH2 proficiency testing (PT) program administered by the College of American Pathologists (CAP). The Survey began in 1996 as a pilot survey and now includes three fresh pooled whole blood specimens (at three HbA1c levels). Samples are shipped on cold packs using an overnight courier. Target values for each specimen are assigned by the mean result of all SRLs in the NGSP network. Proficiency testing data are used to assess the effectiveness of the standardization program by: 1) estimation of bias from the target (by laboratory, by method, by method type, and including all HbA1c methods), 2) interlaboratory comparability within and between methods, and 3) CV of all laboratory results. Beginning in 2007, NGSP target values are used for laboratory grading by the CAP.