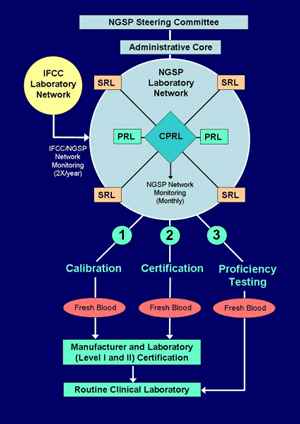
NGSP Protocol
 |
View/Print Entire Protocol (pdf)
Overview | Steering Committee | Laboratory Network | Certification | Proficiency Testing
Certification
C. Manufacturer Certification
1. Standardization of Methods Prior to Certification of Traceability: The process by which a method is standardized to the Reference will depend on the specific method and is determined by the manufacturer, e.g. standardization may be accomplished by re-assignment of calibrator values or by conversion equation since the method may or may not include calibration by the end user. Manufacturers may request assistance from the any network laboratory to 1) help determine the best approach to standardization, 2) recommend or to evaluate different calibrator materials 3) assign preliminary values to calibrators with the understanding that an adjustment in the values may have to be made based on results of fresh sample comparisons, and/ or 4) perform analyses of fresh samples to provide the manufacturer with data on the new method's values compared to reference values in order to provide a basis for calibrator value assignments.
2. Certification of Traceability to the Reference Method: Manufacturers desiring certification of their method must contact the NETCORE. The NETCORE will then assign a SRL based on manufacturers’ preference, method type, and/or potential conflicts of interest. If the manufacturer has done a comparison with a particular SRL in the past, they may contact that SRL directly to arrange for samples to be shipped. Method comparison must be performed for certification of a method as traceable to the DCCT Reference. Before pursuing certification through the SRL, manufacturers should establish that their analytical instrument systems / methods:
- have had all required preventive maintenance procedures performed
- be in peak operating condition
- be operated with the same parameters in all runs of the comparison and precision studies (e.g. instrument, reagent lot, calibrator lot, calibrator assigned values)
- be operated in the same manner as they would by a customer
Manufacturers must participate in a fresh or fresh/frozen blood sample comparison (n=40) for estimation of bias from the SRL. Manufacturers may perform the comparison analyses at the manufacturing site, or they may choose to have a laboratory (that is NOT an NGSP network laboratory) using their system perform the testing. For the sample comparison, one set of fresh samples may be split and used to evaluate several applications or methods, thus necessitating only one evaluation by the SRL. Collection of patient samples may be done either by the manufacturer (or designated laboratory) or by the SRL as long as sample stability requirements for both the SRL and the manufacturer’s method can be met. All data should be sent from the manufacturer and the SRL directly to the NETCORE. Along with these results, the manufacturer may be asked to submit raw data that includes date, time, and results for each of the 40 samples. Therefore, these data should be collected when the samples are analyzed.
In order for a commercial method to be considered traceable to the CPRL, 36/40 of the individual results must be within 5% of the SRL means.
All data analysis will be performed by the NETCORE. Outliers will be analyzed for informational purposes only; an outlier is defined as > mean + 3SD of the absolute differences between the test method and the SRL. All outliers will be investigated by the NETCORE to determine if the discrepancy could be due to characteristics of the specimen rather than the assay method. If results show that a discrepancy could be due to characteristics of the specimen, then the manufacturer will be asked to submit a new specimen and the data will be reanalyzed.
Manufacturers are awarded Certificates of Traceability for successfully completing fresh sample comparisons for the specific reagent lots, calibrator lots and instrumentation. Traceability to the DCCT applies only to results from fresh or fresh frozen blood samples. Final certification of traceability is issued by the NETCORE. Certificates of Traceability are valid for one year. Certified methods are listed on the NGSP web site. Methods are required to be re-certified annually to remain listed on the NGSP web site. A new certification is also required in the event of significant changes, such as those that would require a new 510(k) form to be filed with FDA.
In the event that a method fails certification, the manufacturer must investigate the root cause(s) of the failure, document the reason(s) for the failure and describe the corrective measures implemented to prevent a failure in the future prior to a subsequent attempt to obtain certification. In addition, a description must be provided how these changes will be communicated to and applied to the methods in use by end-users. If the method is intended for use at the point of care, resubmission following a failure must include data for 3 different reagent lots. The passing criteria must be fulfilled separately for data from each reagent lot to be certified on a resubmission.
If a second attempt to certify is not successful, the manufacturer cannot resubmit for another attempt at certification until one year from the date of the notification of failure.
D. Level I Laboratory Certification
Level I Laboratory Certification includes both yearly certification of traceability to the DCCT and a quarterly monitoring component to insure stability throughout the year. This type of certification is recommended for large laboratories that are involved in research studies or clinical trials where long term precision of glycohemoglobin measurement is critical, i.e. where a small shift in glycohemoglobin results could affect the final outcome of a trial or research study. The certification protocol for Level I Laboratory Certification will be the same as followed for manufacturer method certification as outlined in section C. However, the certification criteria are more stringent; 37/40 of the individual results must be within 5% of the SRL means.
Laboratories are awarded Certificates of Traceability for successfully completing fresh sample comparisons for the specific method, reagent lots, calibrator lots and instrumentation used. Traceability to the DCCT applies only to results from fresh or fresh frozen blood samples. Analysis of processed (e.g. lyophilized) material may be subject to matrix effects and any comparisons to the DCCT using results from processed specimens should be made with caution.
Level 1-certified laboratories are also monitored on a quarterly basis using 10 fresh samples, analyzed in two separate runs on two separate days. To maintain certification a level I laboratory must fulfill the following bias and precision requirements: mean difference between laboratory and the mean of the SRLs ≤0.30% HbA1c, SD of the difference in sample replicates ≤0.190. Level I Laboratories must submit quarterly monitoring data within 2 weeks of receipt of monitoring samples and pass the criteria in order to maintain their certification status.If a Level 1 laboratory either does not pass the monitoring criteria, or fails to send in data, for two consecutive quarters, the laboratory will be removed from the NGSP Certified Level 1 Laboratory list. If the laboratory then passes the NGSP monitoring criteria in a subsequent quarter, they will be placed back on the Certified Level 1 Laboratory list.
E. Level II Laboratory Certification
Laboratories can obtain documentation from the manufacturer of their system's performance and traceability to the DCCT, i.e. documentation of NGSP certification. However, a manufacturer's demonstration of a product's traceability (NGSP certification) does not in itself guarantee the accuracy and precision of that product in the hands of every user (clinical laboratory). Laboratories using methods that are not certified must take primary responsibility for establishing that system's traceability to the DCCT. Therefore, laboratories may also participate with a Network laboratory (SRL) to document traceability through a laboratory certification process. The certification process and the certification criteria for Level II laboratory certification are the same as that for manufacturers as outlined in section C. Laboratories are awarded Certificates of Traceability for successfully completing fresh sample comparisons for the specific method, reagent lots, calibrator lots and instrumentation used. Traceability to the DCCT applies only to results from fresh or fresh frozen blood samples. Analysis of processed (e.g. lyophilized) material may be subject to matrix effects and any comparisons to the DCCT using results from processed specimens should be made with caution.