NGSP Protocol
 |
View/Print Entire Protocol (pdf)
Overview | Steering Committee | Laboratory Network | Certification | Proficiency Testing
B. Laboratory Network
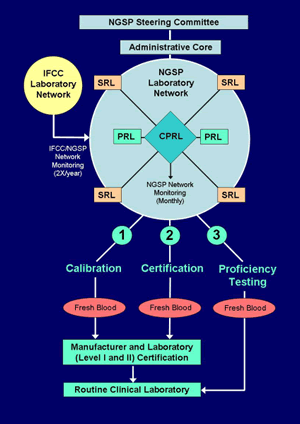
The Network consists of an Administrative Core (NETCORE), a Central Primary Reference Laboratory (CPRL), three Primary Reference Laboratories (PRLs), and seven Secondary Reference Laboratories (SRLs).
1. Administrative Core (NETCORE)
The Administrative Core coordinates the certification process and communicates directly with the Steering Committee. The Core analyzes all certification and network monitoring data, sends reports to the Steering Committee, and issues certificates to laboratories and manufacturers.
2. CPRL
The CPRL analyzes HbA1c by HPLC using Bio-Rex 70 resin following the existing CPRL method protocol and sets the initial calibration for the standardization program based on the "set-point" used in the DCCT. The CPRL must document an accepted level of precision (total imprecision <3%) following NCCLS EP5-A2 guidelines (1). Back-up Primary Reference Laboratories (PRLs) and Secondary Reference Laboratories (SRLs) calibrate their assays so that results from fresh blood specimens agree with the CPRL (see section 2. and 3. below). The CPRL administers a monitoring program for all certified PRLs, SRLs, and Level 1 Laboratories (outlined below in section 5.).
3. Primary Reference Laboratories (PRLs)
PRLs serve as back-up laboratories for the CPRL to ensure that the CPRL function continues without interruption in the event that the CPRL cannot meet the program needs as outlined in section 2. All PRLs analyze HbA1c using the primary reference method according to the CPRL method protocol.
- Primary Reference Method Calibration: A single hemolysate calibrator is prepared by the CPRL; the target value is established by the CPRL based on the mean of at least 50 runs. All PRLs use this calibrator with assigned value to calibrate their instruments with each run according to the CPRL method protocol.
- Primary Reference Method Quality Control: The CPRL prepares hemolysate QC specimens (two levels, a non-diabetic level and a diabetic level of at least 10% HbA1c) following the same protocol as for the calibrator preparation. Each PRL must establish their own QC mean and range criteria and accept or reject assays following the CPRL QC guidelines.
- Certification of Traceability: A proposed PRL must perform precision testing following NCCLS EP5-A2 guidelines (1). The proposed PRL must also participate in a fresh blood sample comparison (n=100) for estimation of bias from the CPRL following modified NCCLS EP9-A2 guidelines (2). For bias estimation, fresh specimens are collected by either the proposed PRL or the CPRL and shipped to the other party. All data for calculation of precision as well as estimation of bias are submitted to the CPRL for analysis.
In order to be considered traceable to the CPRL: 1) total imprecision (CV) must not be statistically significantly >3%, 2) one within-method outlier is acceptable; one between-method outlier is acceptable, 3) 95% confidence interval for predicted bias should overlap the + 3% range of the CPRL at 2 levels (6 & 9% HbA1c). All data analysis is performed by the NETCORE following NCCLS EP5-A2 and EP9-A2 guidelines. If criteria are not met, the proposed PRL may make changes as necessary, re-analyze the same set of samples from frozen aliquots and send the new set of data to the NETCORE for re-evaluation. Final certification of traceability is issued by the NETCORE.
4. Secondary Reference Laboratories (SRLs)
SRLs work directly with manufacturers to assist them in standardizing their methods and in providing comparison data for certification of traceability as outlined in section C. Each SRL analyzes HbA1c by a precise method (documented total imprecision ≤2%) which can be calibrated to the Primary Reference Method. A proposed SRL must demonstrate traceability to the CPRL method.
Secondary Reference Method Calibration: Calibrator may be obtained from a manufacturer or prepared by the proposed SRL. Calibrator values for SRLs are either assigned by the CPRL (by direct analysis or based on fresh sample comparisons with the CPRL) or by the SRL method while calibrated with the initial or previous lot of calibrator.
Secondary Reference Method Quality Control: Each SRL prepares or purchases their own QC specimens (two levels; a non-diabetic level and a diabetic level of approximately 10% HbA1c) and sets their own limits and acceptance criteria using appropriate QC guidelines.
Certification of Traceability: A proposed SRL must perform precision testing following CLSI EP5 guidelines (1). The proposed SRL must have also successfully participated in at least four consecutive NGSP quarterly monitoring comparisons (10 samples each) as a certified Level 1 laboratory. All data for calculation of precision and monitoring are submitted to the NETCORE for analysis.
Secondary Reference Method Certification Criteria:
- 20 day precision evaluation following EP5 using two fresh-frozen single use aliquots of WB, one in the normal non-diabetic range (HbA1c ~5%; 31 mmol/mol) and one in the diabetic range (HbA1c: ~9-10%; 75 – 86 mmol/mol). Total imprecision (CV) must not be statistically significantly >2%.
- Comparison to NGSP network SRLs: 40 of the most recent previous quarterly monitoring (MMON) samples using single results (first of each duplicate) compared to the mean of SRL results.
- 37 of 40 results must be within ±5% (relative) of the SRL mean.
- For each quarterly monitoring comparison, candidate method results must also fall within a defined acceptance ellipse based on the slopes and intercepts of the differences between the individual SRLs results and the medians of all SRLs.
All data analysis is performed by NETCORE. If the criteria are not met, the proposed SRL may make changes to their method as necessary and provide documentation of these changes to NETCORE for approval to repeat the certification process. Final certification of traceability is issued by the NETCORE.
Requirements for new SRLs
Candidate SRLs that have not previously participated in the NGSP network must be able to fulfill the following requirements:
- Must be able to participate in monthly monitoring (10 samples/month) by CPRL.
- Have experience with and be able to communicate with interested manufacturers and laboratories without conflicts of interest.
- Must be able to collect and analyze certification samples according to the NGSP protocol, which includes screening for Hb variants and elevated HbF (>2%), and provide them to manufacturers and laboratories upon request.
- Be able to send invoices to manufacturers and laboratories participating in the certification process following NGSP fee schedule and pay NGSP the data analysis portion upon receipt of NGSP (University of Missouri) invoice. Rates for payment to NGSP are $350 for each manufacturer and level II laboratory certification, $1850 for each level I laboratory certification.
- International (non-U.S.) laboratories must provide NGSP with documentation that they have the support of the professional clinical community in their country.
- Depending upon shipping logistics/costs, international laboratories may need to provide some or all of the costs of shipping monthly monitoring specimens to their laboratory.
Consideration for Network membership will be given to the laboratory’s geographic location and the need for an additional network laboratory. The NGSP Steering Committee reserves final authority in determining which laboratories are eligible to participate in the Network.
5. Performance Monitoring of Primary and Secondary Reference Laboratories
Monthly surveys are administered by the CPRL to monitor performance of the network Primary and Secondary Reference Laboratories. Each month, the CPRL ships 10 specimens (fresh or fresh/frozen whole blood) over the desired clinical range (4-10% HbA1c) to each network laboratory within 2 days of collection. The CPRL and/or each PRL prepares hemolysates following the CPRL sample preparation procedure (aliquots of each hemolysate may be frozen at -70oC or colder prior to assay). Each SRL prepares samples following their method protocol. The CPRL, PRLs and SRLs analyze the specimens in two separate runs on two separate days. All data is sent to the NETCORE for analysis. Each Level I-certified laboratory receives monitoring specimens quarterly.
To maintain certification, a network (PRL and SRL) laboratory must fulfill the following bias and precision requirements: 1) the mean of the differences (n=10) between the network laboratory and the CPRL must not exceed 0.30% HbA1c and 2) the estimate of the standard deviation of the difference in sample replicates must not exceed 0.190 (99th percentile of the sampling distribution around a target SD of 0.125). SRL results must also fall within a defined acceptance ellipse based on the slope and intercept of the differences between the individual SRLs results and the medians of all SRLs. In addition, results for individual specimens where the within-SRL replicates differ by >0.3% HbA1c or the mean result differs from the median of the SRLs by >0.4% HbA1c will be excluded from the final SRL mean calculations. All results outside these limits are investigated. All monitoring results are shared among network laboratories.
6. Monitoring of the relationship between the IFCC and NGSP Networks
Twice each year five frozen whole blood specimens are analyzed by both the NGSP and IFCC networks. Each year the relationship between the networks is compared to the master equation which is based on eight comparison studies over a four-year period. A detailed statistical analysis is performed to insure that the relationship has not significantly deviated from the master equation.
References:
- NCCLS. Evaluation of Precision Performance of Quantitative Measurement Methods; Approved Guideline;Second Edition. NCCLS document EP5-A2 (ISBN 1-56238-542-9). NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2004.
- NCCLS. Method Comparison and Bias Estimation Using Patient Samples; Approved Guideline;Second Edition. NCCLS document EP9-A2 (ISBN 1-56238-472-4). NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2002.